I love science! I love doing experiments with my kids at home and teaching science at school. Children are naturally curious about the world around them and are eager to explore. The amount of learning and excitement that happens with a great experiment makes me very happy. I have collected over 80 science experiments for kids to try out at home, or in the classroom.
My main goal with science experiments are to encourage a love of science and learning in the children who do them. Most of the ideas in this collection are simple hands-on experiments that explore scientific concepts in kid friendly ways.

The experiments may not always work out perfectly every time, but that is a huge part of science and experimenting.
Search through the list to find the perfect easy science experiments to do at home or in your classroom today. I hope that you have as much fun as we do with the experiments!
Most of the science experiments below use kid friendly materials and hands-on exploration. However, as with all science experiments, adult supervision is necessary at all times. Make sure to choose activities that are appropriate for the age and abilities for the children you are doing the experiment with.

81+ Easy Science Experiments for Kids
Click the image to view the science experiment description.
Easy Science Experiments with Water
#1
A few materials create these simple Fireworks in a Jar. Kids will love the ‘explosion’ as they learn about science.
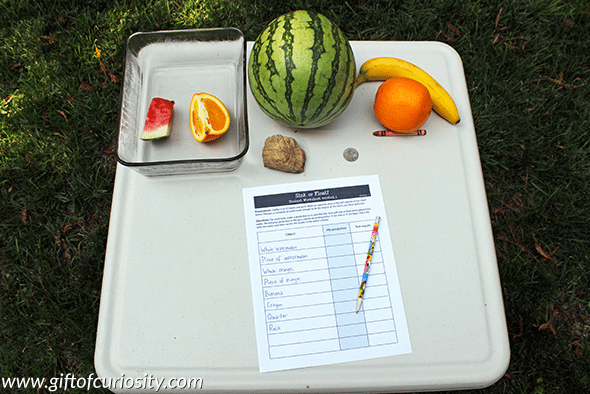
Use water balloons to try out the sink float experiment in a whole new way!
Kitchen chemistry that will wow your kids! Can you clean pennies with ketchup? Find out!
Try this “magic” experiment with items in your pantry!
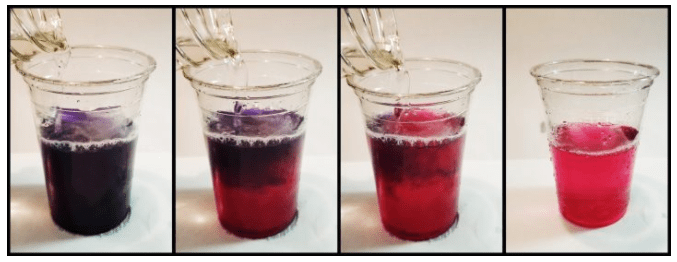
It looks like magic, but it’s science. Starting with purple water, watch the color change before your eyes with science!
This is amazing to watch! Learn about air pressure and rising water.
This is one of my favorite ways to introduce science to children! For this experiment, grab a bunch of sharp pencils and a baggie full of water and the science is guaranteed to amazing little ones.
Just pierce the pencils through the bags one at a time. The pencil seals the bag where it goes through so there’s no mess and no dripping water. That’s all there is to it!
Dancing Beads Experiment
Kids and adults will love watching this dancing beads experiment. A few simple materials will keep the beads moving as kids observe and learn about science and why the beads to what they do.
Make a homemade thermometer using simple materials.
#10 Easy Science Experiments for Kids
Why do leaves change color? This experiment explains it in a kid friendly way.
If you are learning about the human body, creating a “blood’ model is a great way for children to see all of the components of blood!
Create a hurricane that children will love making, playing with and learning about!
Older children can learn about heat conduction and how heat travels from one object to another with this cool science experiment.
You’ve probably witnessed refraction many times, show kids this simple experiment to explain this cool science.
Properties of Water
Learn about the properties of water with this simple science experiment for kids. Look at how molecules travel differently in hot water versus cold water.
This fun and easy Penguin Science Experiment will show your kids how penguins stay dry in the cold temperatures and icy waters.
Kids will learn a little bit about the make up of water and they will love the bubbles with this science experiment.
Kids will love watching the mystery colors appear and mix with this easy to do experiment.
This beach in a jar is a perfect, and beautiful way to teach kids about density.
#20 Cool Science Experiments to do at Home
If you have children interested in learning about weather, this collection of science experiments for kids will answer many of their questions. From the water cycle, rain clouds, and a tornado in a bottle they will love these hands-on experiments.
Can you make the raisins dance? A few simple household materials and your raisins will be dancing in no time!
The colors will disappear before your eyes! Little ones will think it is magic – but it’s science!
This is a great experiment to get rid of some extra candy around the house!
Growing Crystals from Borax is a really fun activity to try at home. Here we show you how to grow crystal stars to hang on your Christmas Tree.
Kids will feel like real scientists with this chemistry experiment as children create layers with different liquids.
Easy Science Experiment for Kids – With Ice
Learn a bit about the water cycle with this fun, hands-on melting snowman science experiment for kids.
How does a polar bear stay warm in the cold? Kids learn how animals have adapted to survive in winter with this hands-on experiment.
Grow your own ice spikes with this science experiment for kids.
This melting ice experiment is perfect for some weekend science to teach little ones about what can cause ice to melt faster.
#30 Easy Science Experiments to do at Home
Use materials you have on hand to create frozen treasures. A free printable scavenger hunt sheet is available to use with the science experiment.
Outdoor Science Experiments for Kids
Learn about rocket science with this kid friendly science experiment.
How do we hear sounds? Experiment with hearing and sound waves in fun and easy ways!
These superheroes show kids all about gravity in a fun, hands-on way.
Why do leaves change color? This experiment explains in a fun, kid friendly way.
Take learning outside with this exciting exploding watermelon experiment.
Have some great outdoor fun learning about bubbles with this collection of Bubble STEM activities!
Try out this tasty science experiment for kids and make smores with a solar oven.
Two ingredients create this explosion that kids will enjoy creating and watching!
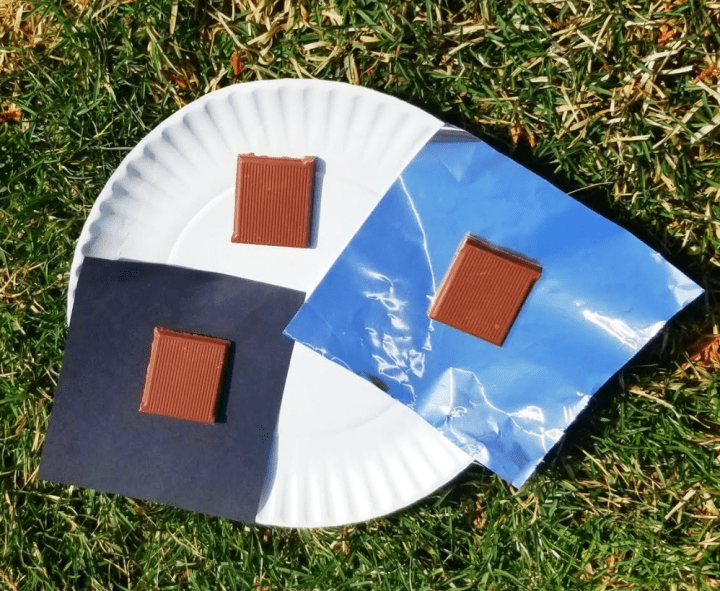
Do you have some leftover chocolate in your house? This experiment shows how fast the sun can melt something and also compares the difference between the shade and direct sunlight.
#40 Cool Science Experiments to do at Home
Don’t just plant seeds in the ground, give kids the opportunity to observe what happens as seeds sprout!
Take children to the garden to learn lots of different science lessons.
Easy Science Experiments to Try at Home
Who knew milk was so amazing? A few household items create this stunning effect!
You can use pumpkin seeds or other decomposable food for this experiment to teach kids how the environment plays a role in food breaking down.
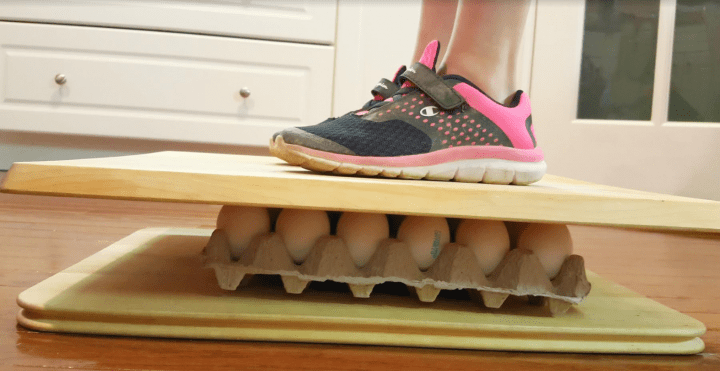
Gather a few dozen eggs and challenge a child, or two, to see if they can stand on the eggs without making scrambled eggs! They’ll love learning the science behind these science experiments for kids.
Ever wondered how flowers and plants ‘drink’ water? Kids do! Clearly show them with this flower experiment.
This twist on the common sink float experiment, tests what effect salt can have when it comes to density.
Create a colorful rainbow with candy and warm water.
Kids test everyday pantry items with iodine with these really interesting science experiments for kids.
The Walking Water science experiment is a wonderful, colorful and fun way to teach kids about absorption and mixing primary colors.
#50
Create a bouncing egg with these egg experiments. Also, teach about dental health using eggs in this creative, but easy science experiment.
Learn about vision and how we see with these awesome eye experiments.
Easy Science Experiments to Try in the Classroom
How do we hear? A few simple science experiments for kids show how sound travels and how we hear.
This is one of my favorite classroom experiments that I have ever done! Use mealworms to show children about life cycles in an exciting way they’ll never forget.
Kids will love this hands-on experiment to teach all about friction.
This is great sink or float experiment to test a collections of everyday objects.
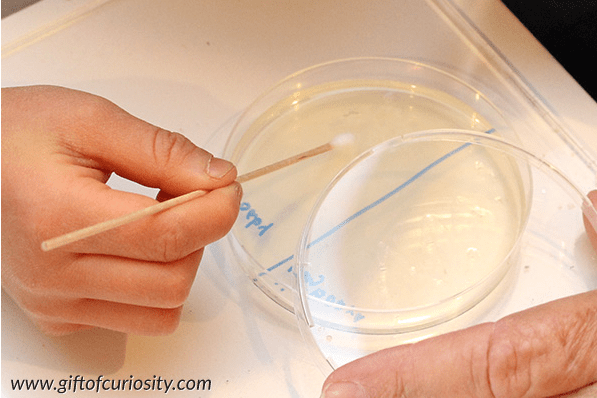
Learn about the bacteria that is all around us, with this hands-on science experiment.
Want to learn how our lungs work? This model made from at home materials shows how!
Build a heart model that really pumps blood!
Teach children about cells with this fun cell study using eggs!
#60 Science Experiments at Home for Kids
If you’re learning about the human body, this experiment and model of the ear is a great learning experience.
If you are looking for quick and easy, but exciting science experiments for kids, try out this oil and water experiment.
There is so much to learn about rocks and the earth. These hands-on experiments will teach kids all about erosion and weathering.
Test the characteristics of different liquids and solids with these hands-on experiments. Perfect for the classroom or homeschooling.
Learn about air pressure with these science experiments for kids.
Simple Science Activities – Exploding Science
Mix a bit of art and science with this clay volcano that children create and can then use to make their own volcanic ‘explosion’.
Can you inflate a balloon without blowing into it? With science you can!
How beautiful is this lava lamp? Kids will love making, and then watching their lamp.
A fizzing rainbow! Kids will love this beautiful, simple science experiment.
Use a few simple household materials, to create this erupting lemon volcano chemistry experiment!
#70 Easy Science Experiments
Kids and adults will love to watch this foaming experiment!
With a few simple ingredients found in most households, these hand made disks are perfect for any season or holiday. Find the treasure inside while learning about chemical reactions.
STEM Challenge Science Experiment
We turn on our tap and get clean water, but it’s not quite that easy. Have children explore a little bit about how water is filtered and clean with this hands-on STEM challenge and science experiment.
Challenge children to create a structure to hold a mini pumpkin, or other object, and then try a sink or float experiment.
This is such a cool, and simple experiment to teach kids about the effects of earthquakes.
Try something new this summer by creating a water compass.
Easy Science Experiment – Sensory Activities
If you have never created oobleck, this magic color changing oobleck is a great hands-on science experiment for kids.
Get a bit messy with these beautiful hand made moon rocks and science experiment.
Explore the idea of cold blooded and warm blooded with this really interesting science experiment that will help children understand the difference.
Easy Science Experiments – Art + Science
Kids will love this rainbow colored experiment. It is easy to prepare and exciting to do.
#80 Cool Science Experiments to do at Home
This science experiment for kids mixes a bit of art and science and is perfect for young scientists and outdoor fun.
Kids will love creating their own volcano and then making it explode!
Bonus Ideas
Borax crystal flowers are the perfect science craft!
Create gorgeous crystal formations with this simple, hands-on experiment.
For more ideas of how to integrate science into your child’s learning program, check out these ideas for the best Free Homeschool Science curriculum.
For your convenience, this post contains affiliate links. As an Amazon Associate I earn from qualifying purchases and I may earn a small commission at no cost to you.
Join Hands-On Teaching Ideas
Join Hands-On Teaching Ideas to gain access to my Free Resource Library filled with lots of printable learning resources, from a choice board full of STEM activities and science experiments for kids to escape room games, you can download anything that interests you for your classroom or home. Subscribe here.
More Hands-On Teaching Ideas
If you are looking for more activities and ideas to do in the classroom, or at home with kids, below are some of my favorite and most popular learning activities.
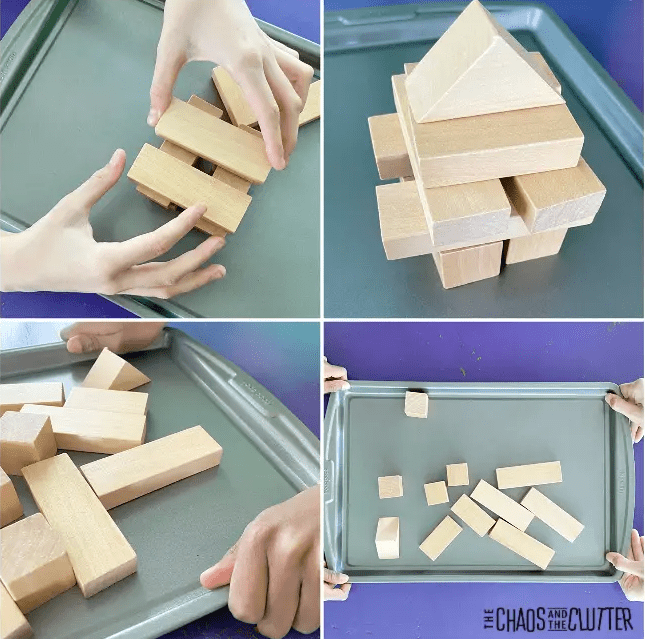
From a STEM escape room game and STEM building challenges that kids can create at home to experiments and challenges with water there are lots of things to keep kids busy and learning. Click image for activity description.

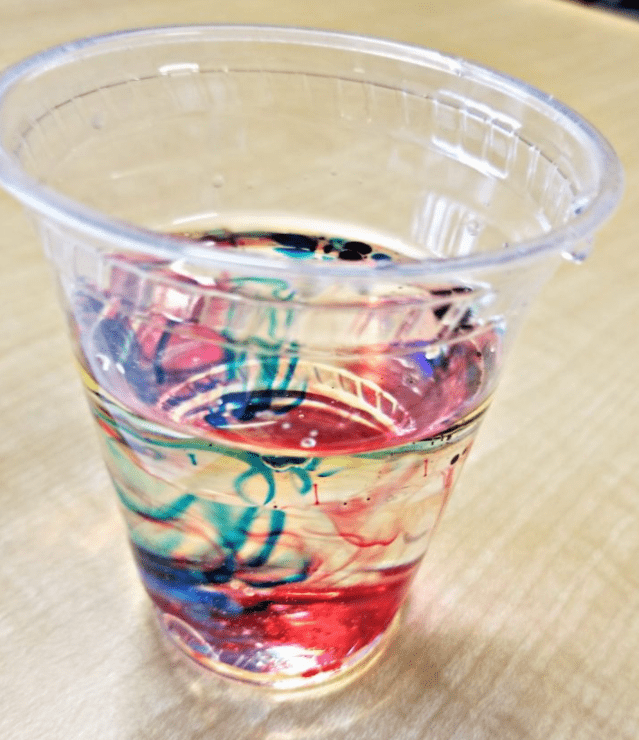





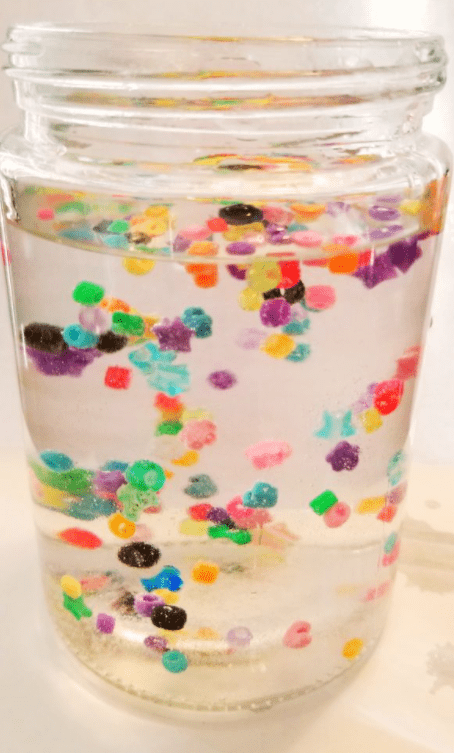

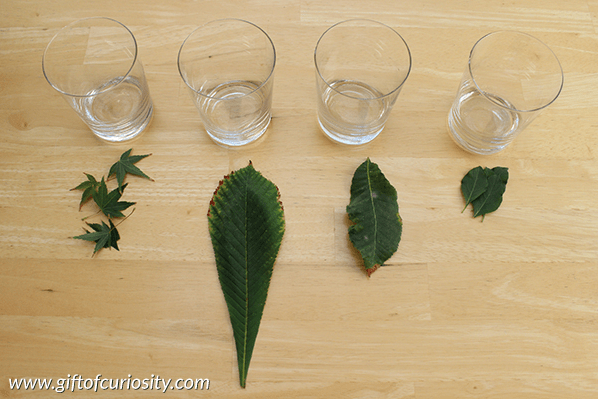


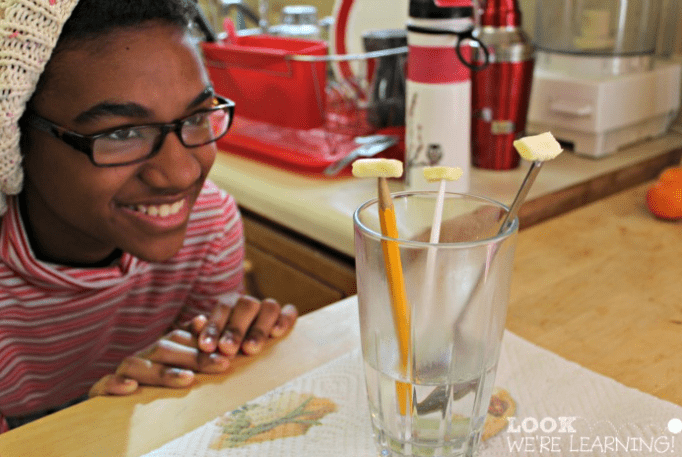




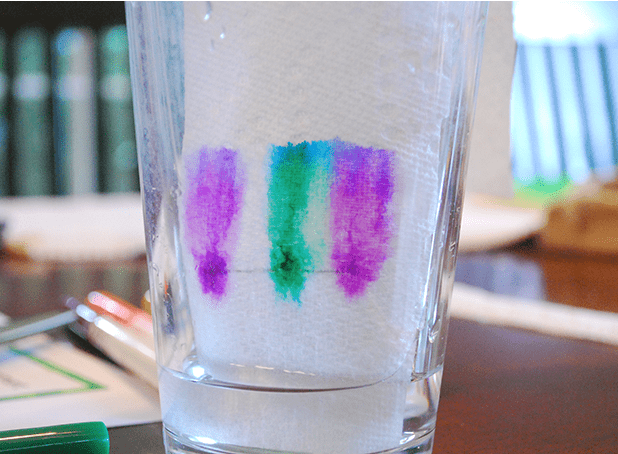











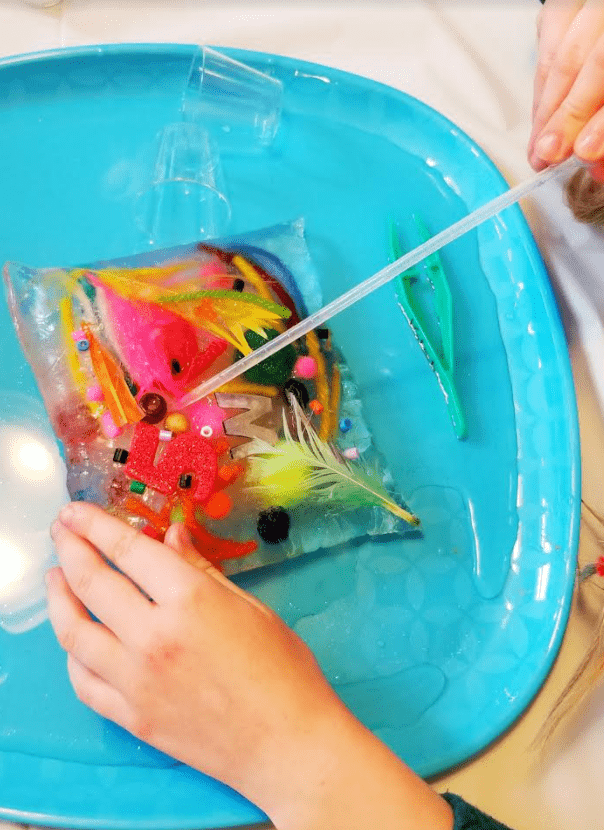





















































Leave a Reply